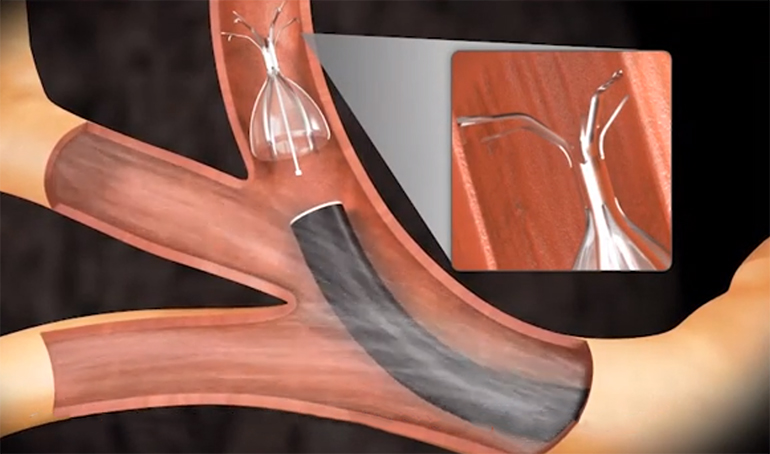
Olympus landed FDA approval for its Spiration Valve System, a product designed to treat people suffering from severe emphysema. It’s used to perform bronchoscopic lung volume reduction (BLVR) procedures, which channel air away from hyperinflated portions of the lungs to healthier areas. In many patients this results in easier breathing and considerably improved quality of life.
The implantation procedure is minimally invasive and the flexible valve can be positioned in pretty challenging airways, including in the upper lobe segments.
The system has European approval already for treating of air leaks and severe, heterogeneous emphysema with evidence of low collateral ventilation such as complete fissures.
Here’s an animation showing the implantation process:
[embedded content]
Product page: Spiration Valve System…
Via: Olympus…
