According to the World Health Organization, 47% of childhood deaths worldwide occur in the first four weeks of life. This neonatal mortality rate is particularly prevalent in sub-Saharan Africa, where nearly one million newborns die every year.
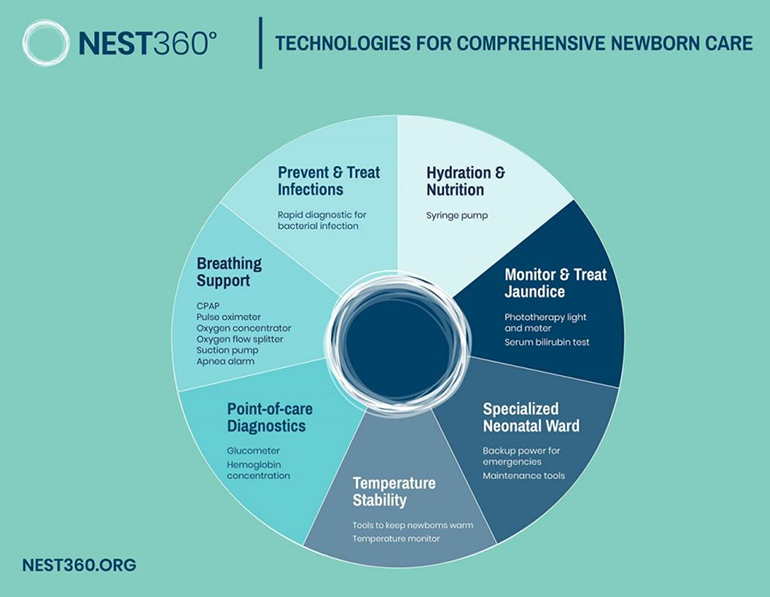
Many of these deaths can be prevented with medical devices that more developed countries often take for granted, such as continuous positive airway pressure (CPAP), phototherapy lights, and temperature monitors. However, solving the problem is not as simple as donating equipment; these devices are often too complicated to operate by limited staff, too resource-intensive to use, or too expensive to maintain, and often times they end up unused and collecting dust.

Rice University and other partner institutions realized that to reduce neonatal mortality in Africa with sustained, long-term efforts, they needed a new approach to developing lifesaving medical equipment and implementing it within clinics. In 2019, NEST360° was started between institutions in six different countries, and the NESTech bundle was developed and implemented at clinics in Malawi, Kenya, Tanzania, and Nigeria.
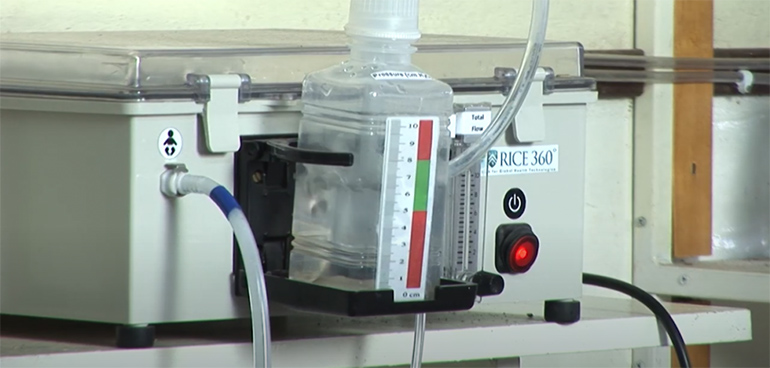
During our recent trip to Houston, we had the opportunity to visit the NEST360° offices at Rice University and see the developmental prototypes of some of the NESTech devices, including the Pumani bubbleCPAP.
We wanted to learn more about NEST360°, and in light of the shortage of medical equipment we’re experiencing due to COVID-19, how some of the medical equipment design principles for Africa could apply to medical product development for the rest of the world. Dr. Maria Oden, a bioengineering professor and co-director of NEST360° at Rice, was kind enough to share some of her thoughts.
Scott Jung, Medgadget: How did NEST360° start, and how did it eventually expand to a team from six different countries?

Dr. Maria Oden, Rice 360° Institute for Global Health: When the Rice team formed, we had developed a bubble CPAP and were working to implement it in all of the government central and district hospitals in Malawi. When we did this we had positive outcomes, but they were not as good as we had seen at the Central hospital where we had conducted our pilot clinical trial. We realized that a single technology in isolation was not going to fully solve the problem. We needed a systematic approach to tackling all the gaps, and so we added experts in all the gaps/challenges we faced and formed a multi-national team for the MacArthur 100&Change competition in 2017.
Medgadget: How does NEST360°’s approach to product development differ from a typical medical device company?
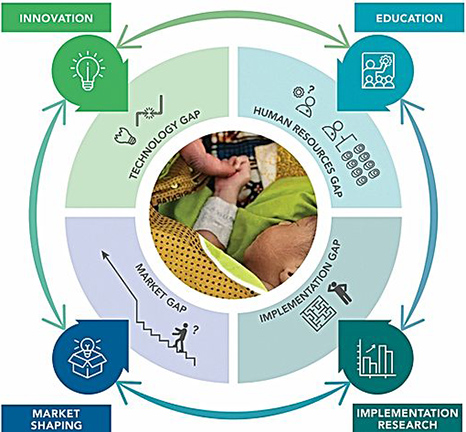
Maria Oden: Our approach is to deeply understand the environment where the technology will be used and design products that allow the caregivers in that environment to successfully treat their patients. This includes making sure the products are easy to use AND repair locally, rugged, and, of course, effective. The challenge is that the market for neonatal products is fairly small and the distribution challenges are quite large, so many of the major medical product manufacturers are less interested in meeting that target market need.
Medgadget: What do you think makes Rice University such a special place for NEST360°?
Maria Oden: The Rice University team members are biomedical engineers, and so they know how to bridge the gap between the product manufacturers and the clinicians in the field. While we at Rice lead the NEST360° effort, the NEST program would not be successful without all our partners.
Medgadget: With the rise of COVID-19, we’ve seen many groups help to develop lower-cost, faster-to-manufacture devices to help with the equipment shortage we’re facing. What are some lessons you’ve learned at NEST360° that could also be helpful for these groups to know?
Maria Oden: It is critically important to still think through all the possible safety issues with any product that is being developed. These groups need to understand the use environment as well as the medical standards that need to be met to have a successful and useful product at the end. This is somewhat of a challenge right now in the face of a pandemic, but it remains extremely important as products are developed.

Medgadget: What’s in store for the future of NEST360°?
Maria Oden: We are scaling the NEST program to hospitals in Malawi, Tanzania, Kenya and Nigeria. Our efforts have been slowed a bit due to the pandemic, but we remain committed to working with our partner countries to halve newborn deaths in hospitals in Africa. The newborn is the most vulnerable part of the health system.
The challenges due to COVID-19 are sure to stress the entire health system in Africa – and we must all remain committed to ensuring that babies can be born in facilities that have the necessary health systems and infrastructure, including technologies, in place.

More information: NEST360° and Rice 360° Institute for Global Health
