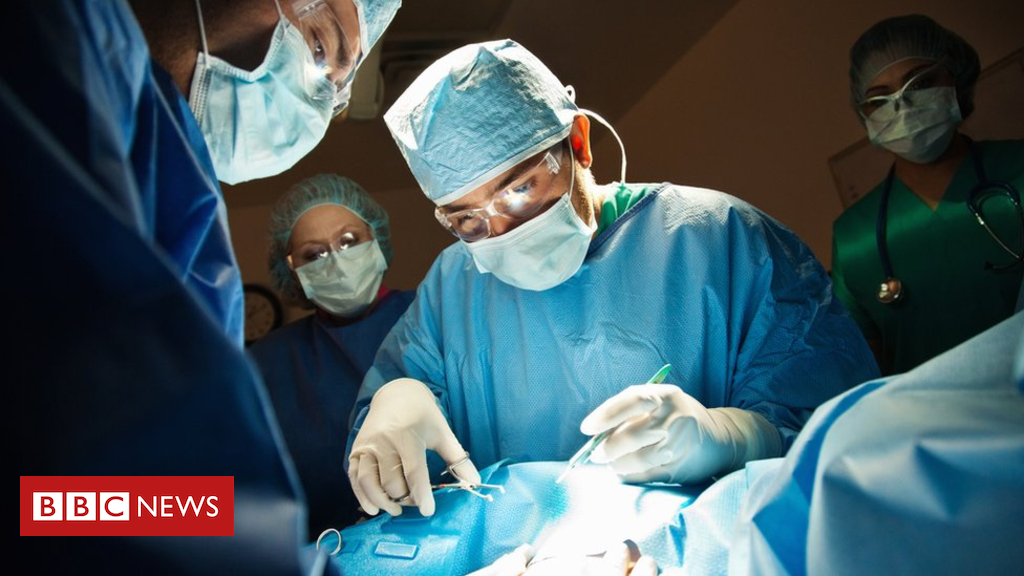
Spinal surgery for unborn babies with the birth defect spina bifida is to be made routinely available on the NHS in England, officials have announced.
The surgery involves repairing the spinal tissue of the baby while it is still in the womb.
It can improve their ability to walk and reduce health problems that result from spina bifida.
The procedure is among several treatments being made available on the NHS for the first time from April.
More than 200 babies are born in the UK each year with spina bifida, where the spine and spinal cord do not develop properly during pregnancy, causing a gap in the spine.
It often results in problems that include paralysis of the legs, incontinence and sometimes learning difficulties.
The condition is usually treated after birth, but the earlier it is repaired the better for long-term health and mobility.
Spinal surgery in the womb was carried out in the UK for the first time earlier this year on two unborn babies at University College Hospital in London.
In the past, patients had to travel abroad for the procedure.
It is not known what causes spina bifida but a lack of folic acid can increase the risk.
Kate Steele, chief executive of charity Shine, said: “Although open pre-natal surgery is not a cure for spina bifida, and is not suitable for every pregnancy, any medical advances which will potentially improve the health and social outcomes for a baby born with spina bifida is very good news, and Shine welcomes this progress.”
‘Life-changing treatment’
Among the other treatments that will be routinely offered on the NHS is the drug everolimus for epileptic seizures caused by a genetic condition that results in benign tumours developing in the body and brain, known as tuberous sclerosis complex (TSC).
More than 300 people, mostly children, will benefit from this new treatment that reduces the number and severity of seizures.
Louise Fish, chief executive of the Tuberous Sclerosis Association, said: “We’re delighted that NHS England has decided to fund this life-changing and potential life-saving treatment from April 2019 onwards.
“We’ll be working with TSC clinics across England to help them get ready to prescribe this drug to more people who can benefit from it.”
The other treatments that will be funded from 1 April 2019 are:
- Small bowel transplantation service (adults) and small bowel transplantation (children)
- Selexipag for pulmonary hypertension
- Trientine dihydrochloride for Wilson’s Disease
- Selective internal radiation therapy (SIRT) for chemotherapy refractory / intolerant metastatic colorectal cancer
- Metreleptin for congenital leptin deficiency
- Gemcitabine and capecitabine as adjuvant treatment for resected pancreatic cancer
